Passage
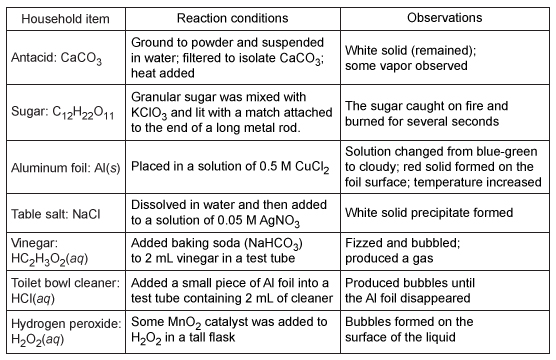
Students were asked to bring items from home to serve as examples for different types of reactions. The instructor provided additional reactants, solvents, heat, or a catalyst to demonstrate reactions with the household items.The students brought aluminum foil, table salt, sugar, baking soda, vinegar, toilet bowl cleaner, hydrogen peroxide, and antacid tablets.Experiment 1Reactions were performed using the household items. The reaction conditions and student observations are recorded in Table 1.Table 1 Reaction Conditions and Visual Observations
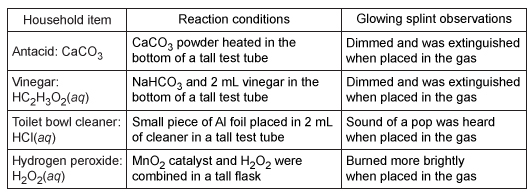
 Experiment 2The reactions that were observed to produce gas bubbles in Experiment 1 were repeated, and samples of the gases produced were collected. Each gas sample was tested by holding a burning (glowing) wooden splint within the reaction vessel to determine if the reaction evolved O2, H2, or CO2. A glowing splint placed in O2 will burn more brightly, one placed in H2 will ignite the gas, and one placed in CO2 will be extinguished. The results of the gas tests are recorded in Table 2.Table 2 Glowing Splint Gas Test Observations
Experiment 2The reactions that were observed to produce gas bubbles in Experiment 1 were repeated, and samples of the gases produced were collected. Each gas sample was tested by holding a burning (glowing) wooden splint within the reaction vessel to determine if the reaction evolved O2, H2, or CO2. A glowing splint placed in O2 will burn more brightly, one placed in H2 will ignite the gas, and one placed in CO2 will be extinguished. The results of the gas tests are recorded in Table 2.Table 2 Glowing Splint Gas Test Observations
-How will the percent yield of the reaction with table salt and AgNO3 be affected if some of the solid precipitate is lost when it is filtered from the solution?
A) The percent yield would be greater than 100% because the mass of the isolated product is greater than the calculated mass.
B) The percent yield would be greater than 100% because the mass of the isolated product is less than the calculated mass.
C) The percent yield would be less than 100% because the mass of the isolated product is greater than the calculated mass.
D) The percent yield would be less than 100% because the mass of the isolated product is less than the calculated mass.
Correct Answer:
Verified
Q181: Liquid nitrogen is used in cryosurgery for
Q182: Passage
Students were asked to bring items from
Q183: If a channel of a particular cell
Q184: Which statement correctly describes both the 3s
Q185: Which of the following carbon-carbon bonds in
Q187: Consider the titration curve for aqueous ammonia
Q188: Given that acetic acid (CH3COOH) has pKa
Q189: In elements 1-86 on the periodic table,
Q190: Shiny nickel metal granules added to orange
Q191: Which of the following molecules has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents