Consider the titration curve for aqueous ammonia shown below. 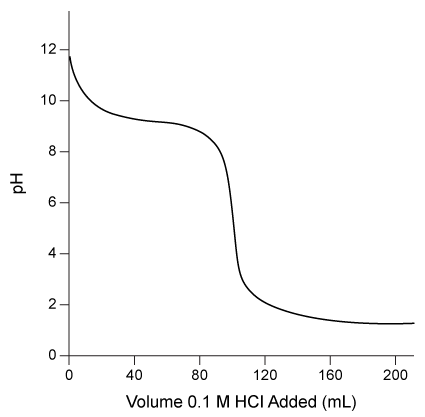 The quantity of hydrochloric acid required to reach the equivalence point in the titration is:
The quantity of hydrochloric acid required to reach the equivalence point in the titration is:
A) 9 mmol.
B) 10 mmol.
C) 12 mmol.
D) 16 mmol.
Correct Answer:
Verified
Q182: Passage
Students were asked to bring items from
Q183: If a channel of a particular cell
Q184: Which statement correctly describes both the 3s
Q185: Which of the following carbon-carbon bonds in
Q186: Passage
Students were asked to bring items from
Q188: Given that acetic acid (CH3COOH) has pKa
Q189: In elements 1-86 on the periodic table,
Q190: Shiny nickel metal granules added to orange
Q191: Which of the following molecules has the
Q192: The radioisotope gallium-68 is used in nuclear
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents