Passage
Amino acids and copper both serve vital roles in many biological processes of living organisms. Beyond their separate biological roles, however, chemists have also discovered that amino acids are able to function as chelates with Cu2+ ions to form blue complexes with potential medicinal applications. One such complex, copper(II) glycinate, has shown to be effective against some oral diseases. The reaction to form copper(II) glycinate is shown in Figure 1.
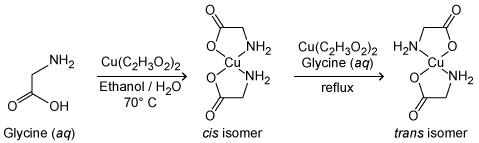 Figure 1 Synthesis of two isomers of copper(II) glycinateTwo different isomers can form depending on the reaction conditions. Mixing hot aqueous glycine (70 °C) with a hot solution of copper(II) acetate dissolved in 50% aqueous ethanol, followed by cooling the mixture on ice, yields a precipitate of only the cis isomer. If a suspension of the cis isomer is held at boiling for an hour or longer in a solution of glycine and copper(II) acetate, the trans isomer is obtained.Because of the geometric differences between the two isomers, the stretching vibrations of the central Cu-N and Cu-O bonds in each isomer are different. Symmetric and asymmetric bond stretching give four possible vibrational modes for each isomer, as shown in Figure 2.
Figure 1 Synthesis of two isomers of copper(II) glycinateTwo different isomers can form depending on the reaction conditions. Mixing hot aqueous glycine (70 °C) with a hot solution of copper(II) acetate dissolved in 50% aqueous ethanol, followed by cooling the mixture on ice, yields a precipitate of only the cis isomer. If a suspension of the cis isomer is held at boiling for an hour or longer in a solution of glycine and copper(II) acetate, the trans isomer is obtained.Because of the geometric differences between the two isomers, the stretching vibrations of the central Cu-N and Cu-O bonds in each isomer are different. Symmetric and asymmetric bond stretching give four possible vibrational modes for each isomer, as shown in Figure 2.
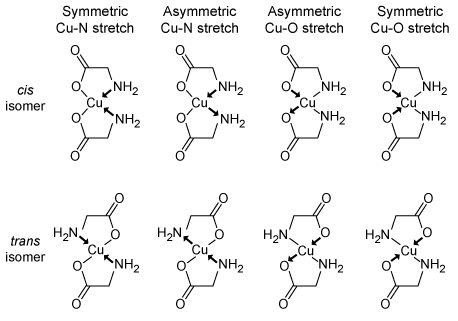 Figure 2 Vibrational modes of the coordinate bonds in copper(II) glycinateIf the stretching of the bonds during vibration causes a transient, unequal distribution of electron density between the atoms within the bond, an oscillating dipole will form. Bonds with dipoles that are not cancelled out by another dipole in the molecule will absorb infrared light at particular frequencies (often expressed in wave numbers) and are designated as IR-active. As a result, the vibrational differences between the two isomers can be analyzed by far-infrared (far-IR) spectroscopy, as shown in Figure 3.
Figure 2 Vibrational modes of the coordinate bonds in copper(II) glycinateIf the stretching of the bonds during vibration causes a transient, unequal distribution of electron density between the atoms within the bond, an oscillating dipole will form. Bonds with dipoles that are not cancelled out by another dipole in the molecule will absorb infrared light at particular frequencies (often expressed in wave numbers) and are designated as IR-active. As a result, the vibrational differences between the two isomers can be analyzed by far-infrared (far-IR) spectroscopy, as shown in Figure 3.
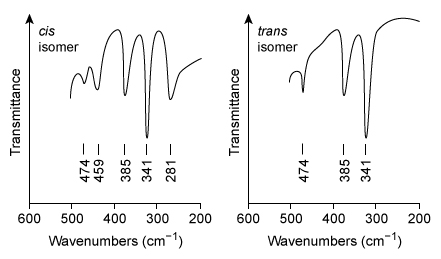 Figure 3 Far-IR spectra of the cis and trans isomers of copper(II) glycinateBoth isomers show a skeletal absorbance (unrelated to coordinate bond vibration) near 385 cm−1. Additional absorbance bands are seen that specifically relate to the vibrational modes of the bonds in each isomer.
Figure 3 Far-IR spectra of the cis and trans isomers of copper(II) glycinateBoth isomers show a skeletal absorbance (unrelated to coordinate bond vibration) near 385 cm−1. Additional absorbance bands are seen that specifically relate to the vibrational modes of the bonds in each isomer.
-In the reaction to form the cis isomer shown in Figure 1, which of the following structural sites function(s) as a Lewis base?
A) Cu only
B) N only
C) O only
D) N and O only
Correct Answer:
Verified
Q223: Passage
Living organisms that require oxygen to respire
Q224: Which diagram below correctly represents the lowest-energy
Q225: Passage
Amino acids and copper both serve vital
Q226: Passage
Within the renal system, two buffers operate
Q227: Passage
Within the renal system, two buffers operate
Q229: Passage
Living organisms that require oxygen to respire
Q230: Passage
Within the renal system, two buffers operate
Q231: Passage
Amino acids and copper both serve vital
Q232: When comparing single, double, and triple covalent
Q233: The conjugate acid of the dihydrogen orthosilicate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents