Passage
Within the renal system, two buffers operate to counter the production of excess H+ from luminal H2CO3. They are located within the tubular lumen, and maintain urinary pH levels between 4.5 and 8.0.The first of these is a phosphate buffer, which serves as the primary buffer for urine because its pKa is very close to extracellular fluid pH of 7.4. The phosphate buffer operates according to Reaction 1. H2PO4− is excreted in urine.
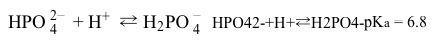 Reaction 1During acidosis when tubular pH is low, the phosphate buffer system becomes saturated. An ammonia buffer system then becomes the primary buffer, and NH4+ is excreted in urine.
Reaction 1During acidosis when tubular pH is low, the phosphate buffer system becomes saturated. An ammonia buffer system then becomes the primary buffer, and NH4+ is excreted in urine.
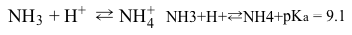 Reaction 2Titratable acid (TA) is the total acid in urine, and it can be found using Folin's method. Under normal physiological conditions, TA is approximately equal to the amount of phosphate in urine, which is a product of serum phosphate concentration and glomerular filtration rate. This makes TA a good indicator of kidney function.Folin's method involves back titration of a urine sample to a pH of 7.4 using a strong base. In a typical procedure, potassium oxalate is added to remove calcium from the sample prior to titration with 1.0 M NaOH. The titration continues until the solution reaches a pH of 7.4 (measured by a pH meter) . The amount of base required for the titration can then be used to calculate the amount of H2PO4− in the urine sample.
Reaction 2Titratable acid (TA) is the total acid in urine, and it can be found using Folin's method. Under normal physiological conditions, TA is approximately equal to the amount of phosphate in urine, which is a product of serum phosphate concentration and glomerular filtration rate. This makes TA a good indicator of kidney function.Folin's method involves back titration of a urine sample to a pH of 7.4 using a strong base. In a typical procedure, potassium oxalate is added to remove calcium from the sample prior to titration with 1.0 M NaOH. The titration continues until the solution reaches a pH of 7.4 (measured by a pH meter) . The amount of base required for the titration can then be used to calculate the amount of H2PO4− in the urine sample.
-A phosphate buffer system based on Reaction 1 is made that contains 5 M H2PO4− and 0.5 M HPO42−. Will the pH of this system fall within the pH range for urine?
A) Yes; pH = 5.8
B) Yes; pH = 6.8
C) No; pH = 9.1
D) No; pH = 10.1
Correct Answer:
Verified
Q221: Passage
Within the renal system, two buffers operate
Q222: Based on the Pauli exclusion principle, which
Q223: Passage
Living organisms that require oxygen to respire
Q224: Which diagram below correctly represents the lowest-energy
Q225: Passage
Amino acids and copper both serve vital
Q227: Passage
Within the renal system, two buffers operate
Q228: Passage
Amino acids and copper both serve vital
Q229: Passage
Living organisms that require oxygen to respire
Q230: Passage
Within the renal system, two buffers operate
Q231: Passage
Amino acids and copper both serve vital
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents