The bond enthalpies for six selected chemical bonds are shown below. 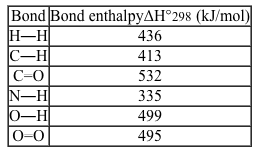 For a reaction in which 2 moles of H2(g) reacts with 1 mole of O2(g) to form 2 moles of H2O(g) , what is the heat of the reaction?
For a reaction in which 2 moles of H2(g) reacts with 1 mole of O2(g) to form 2 moles of H2O(g) , what is the heat of the reaction?
A) 1367 kJ
B) 369 kJ
C) −629 kJ
D) −1996 kJ
Correct Answer:
Verified
Q285: Q286: Which chemical properties below are those of Q287: Passage Q288: Passage Q289: Phosphorus-32 is a radioactive beta-emitter used in Q291: The figure below shows the titration curve Q292: Atoms of solid polonium are packed in Q293: Which of the following equations correctly represents Q294: Passage Q295: During beta decay, a radioactive atomic nucleus![]()
Oxytocin is a naturally occurring peptide hormone
Oxytocin is a naturally occurring peptide hormone
Oxytocin is a naturally occurring peptide hormone
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents