The overall reaction for the electrolysis of molten sodium chloride, 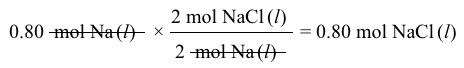 2 NaCll →electric current 2 Nal+Cl2gcan be expressed as two separate, simultaneous redox processes (Reactions 1 and 2) :
2 NaCll →electric current 2 Nal+Cl2gcan be expressed as two separate, simultaneous redox processes (Reactions 1 and 2) :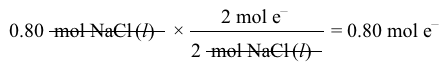 2 Cl-→ Cl2g+2 e-Reaction 1
2 Cl-→ Cl2g+2 e-Reaction 1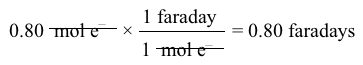 2 Na++2 e-→2 NalReaction 2What is the amount of electric charge required to produce 0.80 mol of Na(l) during the electrolysis?
2 Na++2 e-→2 NalReaction 2What is the amount of electric charge required to produce 0.80 mol of Na(l) during the electrolysis?
A) 0.8 faradays
B) 1.6 faradays
C) 2.4 faradays
D) 3.2 faradays
Correct Answer:
Verified
Q305: What is the mass percent of alcohol
Q306: If a naturally occurring sample of an
Q307: At the end of the actinium nuclear
Q308: A sodium atom in an excited state
Q309: Manganese-54 can undergo a β− decay that
Q311: 4 NH3(g) + 5 O2(g) → 4
Q312: The compressibility of a real gas and
Q313: The boiling point of CH3CH2Br (bromoethane) is
Q314: Which of the following compounds can participate
Q315: The C=S double bond in carbon disulfide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents