The compressibility of a real gas and an ideal gas at a temperature of 200 K is shown as a function of pressure in the graph below. 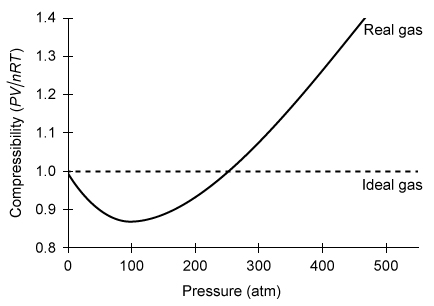 Compared to the volume of the ideal gas at 350 atm, the volume of an equimolar amount of the real gas under identical conditions is:
Compared to the volume of the ideal gas at 350 atm, the volume of an equimolar amount of the real gas under identical conditions is:
A) the same, because the pressure and temperature are the same for both gases.
B) smaller, because the number of moles of each gas is the same but their molar masses are different.
C) larger, because the combined volume of gas molecules is significant relative to the volume of the container.
D) larger, because the intermolecular interactions between gas molecules are significant.
Correct Answer:
Verified
Q307: At the end of the actinium nuclear
Q308: A sodium atom in an excited state
Q309: Manganese-54 can undergo a β− decay that
Q310: The overall reaction for the electrolysis of
Q311: 4 NH3(g) + 5 O2(g) → 4
Q313: The boiling point of CH3CH2Br (bromoethane) is
Q314: Which of the following compounds can participate
Q315: The C=S double bond in carbon disulfide
Q316: Which of the following molecules exhibit an
Q317: What is the approximate molar concentration of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents