Passage
A student performed an experiment to determine the energy content of a sample of potato chips. The chips were blended and then freeze-dried to remove moisture from the sample. Freeze-drying, or lyophilisation, is the preferred dehydration process for food calorimetry due to its minimal impact on nutritional content. Lyophilisation removes water from frozen food samples at low pressure through sublimation. The water is later collected in a cooled condensation chamber to prevent damage to any electric components.After freeze-drying, the sample was ground into a dry homogenous powder. A 1-g sample of the powder was placed in a bomb cell, an enclosed steel-walled container, and pressurized to 25 atm with pure oxygen to ensure complete combustion. The bomb cell was submerged in 500 g of water at room temperature (25°C) in an enclosed and thermally insulated calorimetry device. The sample was combusted using an electric fuse. The temperature of the surrounding water was measured with a glass thermometer every 30 s for several minutes (Figure 1) .
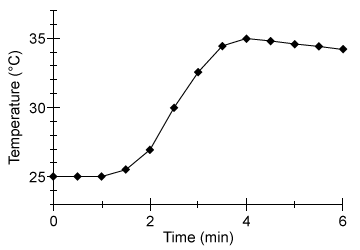 Figure 1 Temperature of the water in the bomb calorimeterThe energy content of food is given in units of dietary calories (Cal) , which is equal to 4.185 kJ, or 1,000 gram calories (cal) . A gram calorie is the amount of energy required to increase 1 g of water by 1°C.
Figure 1 Temperature of the water in the bomb calorimeterThe energy content of food is given in units of dietary calories (Cal) , which is equal to 4.185 kJ, or 1,000 gram calories (cal) . A gram calorie is the amount of energy required to increase 1 g of water by 1°C.
Adapted from Jumpertz R, Venti CA, Le DS, et al., Food label accuracy of common snack foods. Obesity 2013 Wiley-Blackwell.
-Approximately how much heat in kJ was released from the combustion of the 1-g sample?
A) 5 kJ
B) 20 kJ
C) 40 kJ
D) 70 kJ
Correct Answer:
Verified
Q114: Passage
A student performed an experiment to determine
Q115: Passage
A student performed an experiment to determine
Q116: Passage
A student performed an experiment to determine
Q117: Passage
Nitrogen is extremely cold in its liquid
Q118: Passage
Hydrostatic weighing is a technique used to
Q120: Passage
Nitrogen is extremely cold in its liquid
Q121: Two convex lenses are placed in series
Q122: Atmospheric pressure on a distant planet causes
Q123: Relative to the angle of incidence, how
Q124: A sample of water vapor undergoes deposition
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents