Passage
Nitrogen is extremely cold in its liquid phase. It is used in the cryopreservation of small tissue samples and in cryosurgery to freeze and destroy diseased tissues. Liquid nitrogen can be produced from nitrogen gas using the Hampson-Linde cycle (Figure 1) .
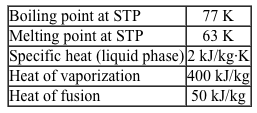
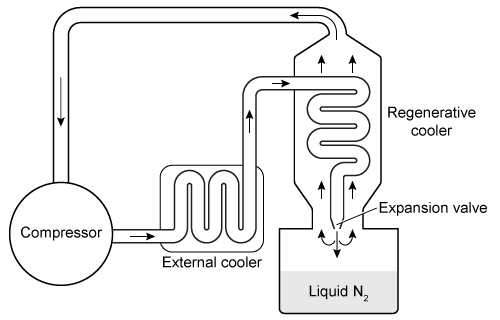 Figure 1 Hampson-Linde cycle apparatus used to liquefy nitrogenThe cycle begins by compressing nitrogen gas and forcing it through a coil in an external cooler that contains dry ice. The gas proceeds to a heat exchanger, where it cools further due to the countercurrent heat exchange with colder nitrogen gas returning from a later stage of the cycle; this process is known as regenerative cooling. The gas is subsequently forced through a narrow valve, where it expands and cools significantly upon exiting. As a result, a portion of the gas becomes liquefied.The temperature change of the gas exiting the valve is described by the Joule-Thomson effect, which occurs without heat transfer with the surroundings. Finally, the liquefied nitrogen is collected, and the remaining gas is sent through the regenerative cooler back to the compressor to reenter the cycle. The physical properties of nitrogen are given in Table 1.Table 1 Thermal Properties of Nitrogen
Figure 1 Hampson-Linde cycle apparatus used to liquefy nitrogenThe cycle begins by compressing nitrogen gas and forcing it through a coil in an external cooler that contains dry ice. The gas proceeds to a heat exchanger, where it cools further due to the countercurrent heat exchange with colder nitrogen gas returning from a later stage of the cycle; this process is known as regenerative cooling. The gas is subsequently forced through a narrow valve, where it expands and cools significantly upon exiting. As a result, a portion of the gas becomes liquefied.The temperature change of the gas exiting the valve is described by the Joule-Thomson effect, which occurs without heat transfer with the surroundings. Finally, the liquefied nitrogen is collected, and the remaining gas is sent through the regenerative cooler back to the compressor to reenter the cycle. The physical properties of nitrogen are given in Table 1.Table 1 Thermal Properties of Nitrogen
-The compressor exerts a pressure of 400 Pa to compress nitrogen gas from 3 L to 1 L. If no heat transfer occurs, how much will the internal energy of the gas change? (Note: 1 m3 = 1000 L.)
A) 0.8 J
B) 1.2 J
C) 800 J
D) 1200 J
Correct Answer:
Verified
Q115: Passage
A student performed an experiment to determine
Q116: Passage
A student performed an experiment to determine
Q117: Passage
Nitrogen is extremely cold in its liquid
Q118: Passage
Hydrostatic weighing is a technique used to
Q119: Passage
A student performed an experiment to determine
Q121: Two convex lenses are placed in series
Q122: Atmospheric pressure on a distant planet causes
Q123: Relative to the angle of incidence, how
Q124: A sample of water vapor undergoes deposition
Q125: Which of the following graphs best illustrates
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents