Which do you think has a higher boiling point, n-butanol or tert-butanol? 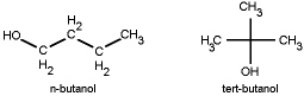
A) n-butanol because it forms stronger hydrogen bonds.
B) tert-butanol because it forms stronger hydrogen bonds.
C) n-butanol because the linear molecule allows for greater dispersion forces.
D) tert-butanol because the tetrahedral molecule allows for greater dispersion forces.
E) they will be exactly the same.
Correct Answer:
Verified
Q19: The process of a solid converting directly
Q20: Given the phase diagram below, at 200K
Q21: The triple point of a compound is
Q22: For a given substance, the vapor pressure
Q23: Which do you think will have the
Q25: In the phase diagram of water increasing
Q26: Dipole-dipole forces are
A) larger than ion-ion forces.
B)
Q27: While many solids are crystalline with a
Q28: Arrange the follow in order of increasing
Q29: The critical temperature for methanol is 512.6
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents