An enthalpy cycle for the formation of monovalent aluminium halides is given below.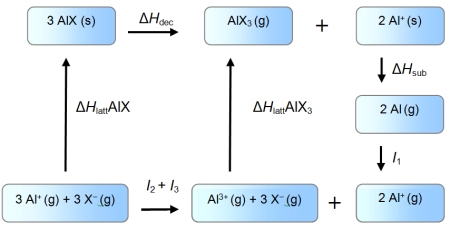
Use the data given below (kJ mol-1) to show which oxidation state is favoured in each case.
I1 = 577 kJ mol-1 I2 = 1816 kJ mol-1 I3 = 2743 kJ mol-1
ΔHsub = 324 kJ mol-1
ΔH (AlF) = -910 kJ mol-1
ΔH (AlI) = -696 kJ mol-1
ΔH (AlF3) = -6380 kJ mol-1
ΔH (AlI3) = -4706 kJ mol-1
A) aluminium(III) fluoride and aluminium(III) iodide
B) aluminium(I) fluoride and aluminium(III) iodide
C) aluminium(I) fluoride and aluminium(I) iodide
D) aluminium(III) fluoride and aluminium(I) iodide
Correct Answer:
Verified
Q11: In the third period (Na to Cl),
Q12: Gallium lies in Group 13 of the
Q13: Unlike iron, aluminium is naturally resistant to
Q14: Why is the C-F bond significantly stronger
Q15: BI3 is a much stronger Lewis acid
Q17: The bridging chlorine atoms in the dimer
Q18: The most abundant oxides of carbon
Q19: Which of the following species does not
Q20: Cyanogen, (CN)2, is sometimes called a pseudohalogen.
Q21: KClO3 and S are used in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents