Sodium chloride, an ionic compound, should be most soluble in which solvent? ( = dielectric constant)
(a) hexane
(b) 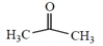
acetone
(c) 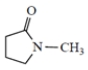
-methylpyrrolidone
(d) 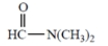
(e) 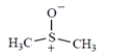
dimethylsulfoxide
A) compound a
B) compound b
C) compound c
D) compound d
E) compound e
Correct Answer:
Verified
Q3: Select the structure of the compound
Q4: When DMSO dissolves potassium chloride (K+ Cl−),
Q5: When two hydrocarbon molecules (such as hexane),
Q6: Dissolving hexane in water has
A) a large
Q7: The boiling point of pentane is 36
Q9: Arrange these compounds in order of increasing
Q10: In each case, identify the better solvent
Q11: By circling three items below each
Q12: Which compound would form the strongest complex
Q13: In which solvent should NaCl (an ionic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents