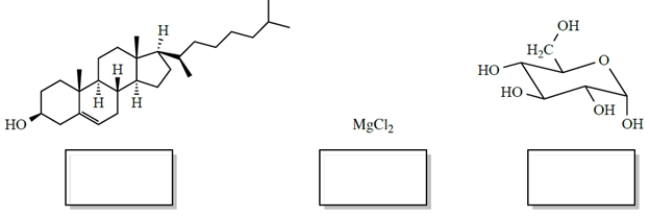
In each case, identify the better solvent for dissolving the compounds. Choose between H2O (ε = 78) or benzene (ε = 2.3). (Benzene is a six-carbon cyclic hydrocarbon.)
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q5: When two hydrocarbon molecules (such as hexane),
Q6: Dissolving hexane in water has
A) a large
Q7: The boiling point of pentane is 36
Q8: Sodium chloride, an ionic compound, should
Q9: Arrange these compounds in order of increasing
Q11: By circling three items below each
Q12: Which compound would form the strongest complex
Q13: In which solvent should NaCl (an ionic
Q14: Until the FDA rescinded the permit for
Q15: Draw the structure of 3-hexyn-2-ol.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents