Consider the acid-base equilibrium:
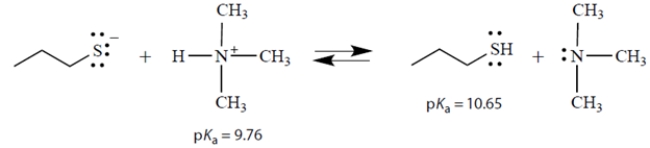 a. What is the equilibrium constant Keq for this reaction?
a. What is the equilibrium constant Keq for this reaction?
b. In the above equation, circle the strongest base.
c. On the left side of the equation, draw the curved arrows that show the formation of the products on the right.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q8: Determine the standard free-energy change of a
Q9: Complete the electron-pair displacement reaction by showing
Q10: Aspirin has the structure on the left
Q11: In this acid-base equilibrium, the pKa
Q12: In the reaction, CH3O− acts as a
Q14: The dissociation reaction for an acid has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents