Select the strongest acid.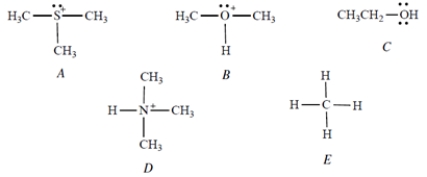
A) Compound A
B) Compound B
C) Compound C
D) Compound D
E) Compound E
Correct Answer:
Verified
Q10: Aspirin has the structure on the left
Q11: In this acid-base equilibrium, the pKa
Q12: In the reaction, CH3O− acts as a
Q13: Consider the acid-base equilibrium: Q14: The dissociation reaction for an acid has Q16: Select the correct statement about this Q17: Equal amount of compounds A and B Q18: Pivalic acid, which has the structure (CH3)3C-CO2H, Q19: Pentothal is a barbiturate used (as its Q20: Choose the number that is closest to
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents