Pentothal is a barbiturate used (as its sodium salt) as a short-acting anesthetic. Pentothal is a weak acid with a dissociation constant Ka = 4 × 10−8 M. Which one of the statements best describes the dissociation state of pentothal in the blood, which has a pH of 7.4?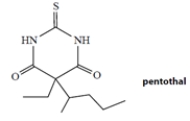
A) more than 90% dissociated
B) between 60% and 90% dissociated
C) about half dissociated
D) between 10% and 40% dissociated
E) less than 10% dissociated
Correct Answer:
Verified
Q14: The dissociation reaction for an acid has
Q15: Select the strongest acid. Q16: Select the correct statement about this Q17: Equal amount of compounds A and B Q18: Pivalic acid, which has the structure (CH3)3C-CO2H, Q20: Choose the number that is closest to Q21: Select the equilibrium that lies farthest to Q22: The free energy of formation (ΔGf°) is Q23: Select the equilibrium that most favors Q24: Predict the approximate equilibrium constant (as![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents