The free energy of formation (ΔGf°) is defined analogously to a heat of formation: it is the standard free energy for formation of a compound from its elements in their standard states at 298 K. Consider the following reaction and their associated standard free energies of formation (all in kJ mol−1). Although these values are for the gas phase, they give us some idea of what to expect in solution.
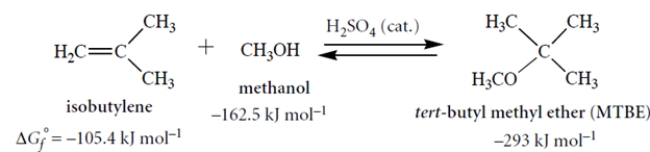 MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
Calculate the equilibrium constant for this reaction in the left-to-right direction. Show your work.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Select the strongest acid. Q16: Select the correct statement about this Q17: Equal amount of compounds A and B Q18: Pivalic acid, which has the structure (CH3)3C-CO2H, Q19: Pentothal is a barbiturate used (as its Q20: Choose the number that is closest to Q21: Select the equilibrium that lies farthest to Q23: Select the equilibrium that most favors Q24: Predict the approximate equilibrium constant (as Q25: Ascorbic acid (Vitamin C) is a diprotic![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents