The rate of formation of the oxime functional group from acetone and hydroxylamine in water is pH dependent. The ideal pH for this reaction is about 4.8.
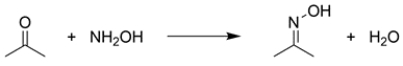 a. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 1.
a. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 1.
b. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 8.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q11: Provide detailed, arrow-pushing mechanisms for the two-step
Q12: Deduce the structure of the missing starting
Q13: Bases can catalyze nucleophilic addition reactions of
Q14: Predict the major organic product for the
Q15: Outline a synthesis for the transformation. Identify
Q17: The conversion of alkyl azides into primary
Q18: Predict the major organic product of the
Q19: Predict the major organic product in the
Q20: Treatment of a mixture of cyclopentanone (A)
Q21: In the reaction of H2O at pH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents