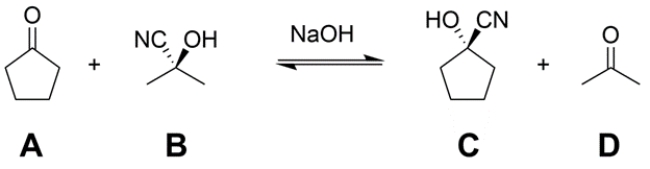
Treatment of a mixture of cyclopentanone (A) and acetone cyanohydrin (B) with a catalytic amount of base (NaOH) leads to an equilibration to form a mixture of A, B, cyclopentanone cyanohydrin (C), and acetone (D). Provide an arrow pushing mechanism for the transformation in the forward direction (A + B → C + D) as written.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Outline a synthesis for the transformation. Identify
Q16: The rate of formation of the oxime
Q17: The conversion of alkyl azides into primary
Q18: Predict the major organic product of the
Q19: Predict the major organic product in the
Q21: In the reaction of H2O at pH
Q22: Draw the structure of the major organic
Q23: Shown are two acetals. In the corresponding
Q24: These two alkenes were prepared by Wittig
Q25: Outline a synthetic route for the transformation:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents