The diene reacts with HBr to give two isomers A and B (C6H11Br).
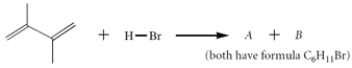 a. Give the structures of A and B.
a. Give the structures of A and B.
b. Give the structure of the carbocation intermediate involved in this reaction. Be sure to show any relevant resonance structures.
c. When compounds A and B are each treated under solvolysis conditions with acetone/water, each compound forms a mixture of the same two alcohols C and D. Give the structures of these two alcohols and explain why both are formed from each alkyl halide.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Which compound should have the greatest
Q2: Which compound has UV absorption at the
Q3: Circle the sp2-hybridized carbons in the structure.
Q5: Which compound or ion has five
Q6: Which compound or ion has a UV/visible
Q7: 1,3-Butadiene, the simplest conjugated diene, has
Q8: All but one of these molecules are
Q9: Which compound has the smaller (less positive
Q10: Select all structures that contain conjugated pi
Q11: Draw the major organic product of this
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents