1,3-Butadiene, the simplest conjugated diene, has a UV absorption at 217 nm. (One nanometer = 10-9 meter.)
a. Calculate the energy of UV radiation with this wavelength (in kJ mol-1). Show your work.
b. Shown is an MO electron-occupancy diagram for the molecular orbitals of 1,3-butadiene. Draw an arrow on this diagram that corresponds to the energy of this UV radiation.
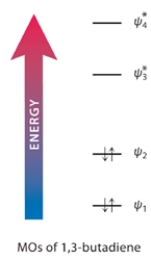
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q2: Which compound has UV absorption at the
Q3: Circle the sp2-hybridized carbons in the structure.
Q4: The diene reacts with HBr to give
Q5: Which compound or ion has five
Q6: Which compound or ion has a UV/visible
Q8: All but one of these molecules are
Q9: Which compound has the smaller (less positive
Q10: Select all structures that contain conjugated pi
Q11: Draw the major organic product of this
Q12: Give the structure of the diene and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents