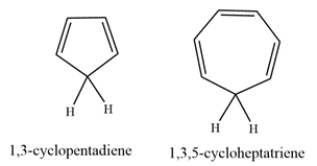
1,3-Cyclopentadiene is unusually acidic, with a pKa of ~16. On the other hand, 1,3,5-cyclohepatriene is much less acidic, with a pKa of ~36. Explain why the compounds have such differing acidities.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Draw the endo product formed in the
Q16: Deduce the starting materials that would give
Q17: The reaction of an allylic alcohol with
Q18: Draw the thermodynamic and kinetic product for
Q19: Which of these compounds is aromatic?
Q20: Cyclooctatetraene (COT) has eight pi electrons but
Q21: Draw a Frost circle and determine the
Q22: Pyridine and pyrrole are both aromatic nitrogen-containing
Q24: Determine whether each compound is aromatic, antiaromatic,
Q25: An arginine residue and a phenylalanine residue
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents