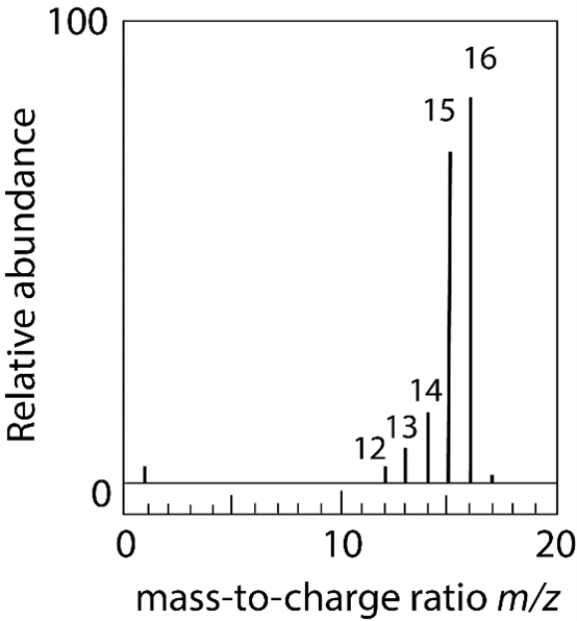
Methane (CH4) is vaporized in a vacuum chamber and bombarded with an electron beam of high energy to give the mass spectrum below. (a) Identify each of the labeled m/z peaks. (b) Explain the presence of the small peak at m/z 17.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: Which molecular vibration does not have an
Q11: The IR absorption that occurs at the
Q12: Identify the compound that corresponds to the
Q13: Use the structure, including the labels on
Q14: Which compound has an absorption at 700
Q16: Which species shown would not be detected
Q17: The base peak of 2,2-dimethylpentane is at
Q18: The electron ionization mass spectrum of 1-hexanol
Q19: These two compounds have similar molecular weights.
Q20: A compound with an unknown structure is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents