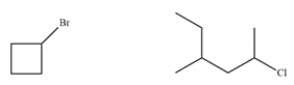
These two compounds have similar molecular weights. Explain how to use mass spectrometry to differentiate between the two.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q14: Which compound has an absorption at 700
Q15: Methane (CH4) is vaporized in a vacuum
Q16: Which species shown would not be detected
Q17: The base peak of 2,2-dimethylpentane is at
Q18: The electron ionization mass spectrum of 1-hexanol
Q20: A compound with an unknown structure is
Q21: The electron ionization of an unknown neutral
Q22: Menthol is obtained from oils of peppermint
Q23: Two students perform a hydrolysis reaction on
Q24: A graduate student is cleaning out the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents