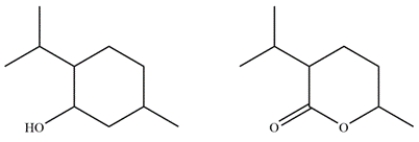
Menthol is obtained from oils of peppermint and spearmint and is often added to cough drops to give a minty, cooling sensation. In the electron ionization mass spectrum of menthol, the molecular ion peak is not observed, but the largest fragment has an m/z 138 at 27% relative abundance and an ion at m/z 139 with a 3% relative abundance. Given the two possible compounds, identify which is menthol and explain how you arrived at that conclusion.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Methane (CH4) is vaporized in a vacuum
Q16: Which species shown would not be detected
Q17: The base peak of 2,2-dimethylpentane is at
Q18: The electron ionization mass spectrum of 1-hexanol
Q19: These two compounds have similar molecular weights.
Q20: A compound with an unknown structure is
Q21: The electron ionization of an unknown neutral
Q23: Two students perform a hydrolysis reaction on
Q24: A graduate student is cleaning out the
Q25: Outline a synthesis for the transformation. Describe
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents