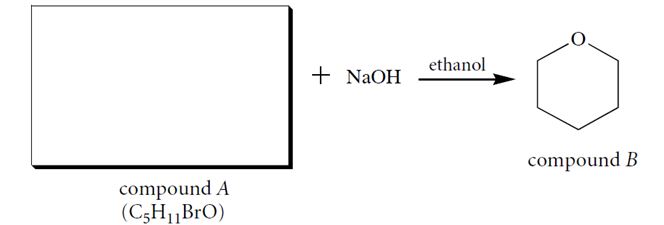
Compound A (C5H11BrO), when dissolved in ethanol containing one equivalent of NaOH, forms a product B. Identify compound A and give a two-step curved-arrow mechanism for its conversion into B. In each step of the curved-arrow mechanism identify the Brønsted acids (BA), Brønsted bases (BB), nucleophiles (N), electrophiles (E), and leaving groups (LG).
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q13: cis-3-Hexene is treated with OsO4 (osmium tetroxide),
Q14: In the SN2 reaction, sulfur is the
Q15: Give the structure of the nucleophile that
Q16: Select the one true statement about biological
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents
