a. Only one of the two diastereomers (formula C12H23OBr) will react with NaOH to form a product X with the formula C12H22O. Identify the reactive diastereomer by circling it and labeling it with the letter A. Draw the product X in its chair conformation.
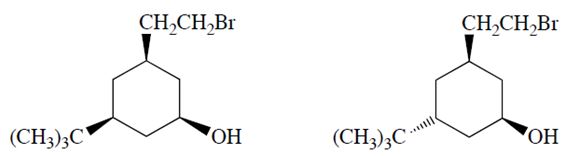 b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
c. Explain why compounds A and B react differently and explain why the formation of X from A is much faster than the formation of Y from B.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Give the structure of the nucleophile that
Q16: Select the one true statement about biological
Q17: Complete the reaction: Q18: Compound A (C5H11BrO), when dissolved in ethanol Q19: Using curved-arrow notation, propose a mechanism for Q21: Draw the missing organic product. Q22: Propose a synthetic scheme to carry out Q23: Propose a multistep synthesis for the epoxide Q24: Complete the diagram by supplying structures for Q25: Outline a multistep synthesis of the given
![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents