Complete the structure for the cyanate ion by adding unshared electron pairs and formal charges. Every atom has an octet, and the overall charge on the ion is −1.
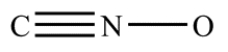
Correct Answer:
Verified
Q2: Which atomic orbital has two nodes?
A) 2p
B)
Q3: Which statement is true about molecular orbitals
Q4: Which one of the following statements about
Q5: How many valence electrons does aluminum (Al,
Q6: What is the formal charge on carbon
Q8: What is the C-N-O bond angle in
Q9: The occupied valence orbitals of the chlorine
Q10: Identify the true statement(s) about molecular orbitals
Q11: Consider this compound: Q12: The atomic orbital that cannot exist is
![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents