Consider this compound:
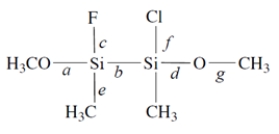 1. Of the labeled bonds, the longest bond is ________________.
1. Of the labeled bonds, the longest bond is ________________.
2. The most polar bond is ________________.
3. The bonding geometry at the silicon atoms is ________________.
4. The hybridization of the silicon atoms is ________________.
Correct Answer:
Verified
2. c
...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q6: What is the formal charge on carbon
Q7: Complete the structure for the cyanate ion
Q8: What is the C-N-O bond angle in
Q9: The occupied valence orbitals of the chlorine
Q10: Identify the true statement(s) about molecular orbitals
Q12: The atomic orbital that cannot exist is
A)
Q13: The lactate ion is a resonance hybrid:
Q14: According to molecular orbital theory, which
Q15: Select the two statements that are true.
A)
Q16: Give the formal charge on the phosphorus
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents