Give the formal charge on the phosphorus atom in this structure. (Phosphorus is in group 5A directly under nitrogen in the periodic table.)
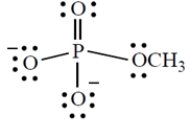
Correct Answer:
Verified
Q11: Consider this compound: Q12: The atomic orbital that cannot exist is Q13: The lactate ion is a resonance hybrid: Q14: According to molecular orbital theory, which Q15: Select the two statements that are true. Q17: Of the bonds marked, which bond is Q18: Given that the acetate anion has the Q19: Add valence electrons to the structures so Q20: What is the geometry of the borohydride Q21: Complete the electron configuration diagram below for
![]()
A)
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents