Complete the electron configuration diagram below for the element boron (B, atomic number = 5), showing 1s, 2s, 2px, 2py, and 2pz orbitals, their relative energies, and their electron populations indicated by "spin arrows" ↑ and ↓.
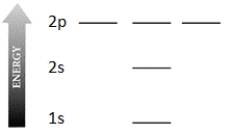
Correct Answer:
Verified
Q16: Give the formal charge on the phosphorus
Q17: Of the bonds marked, which bond is
Q18: Given that the acetate anion has the
Q19: Add valence electrons to the structures so
Q20: What is the geometry of the borohydride
Q22: Assume that the structure of BF3 (boron
Q23: Consider this structure:
Q24: Consider this structure: Q25: In the resonance structures for methyl azide, Q26: Consider this structure:
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents