In the resonance structures for methyl azide, all unshared electron pairs are indicated. Complete the structures by adding the missing formal charges.
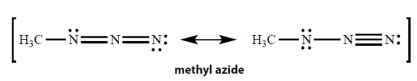
Correct Answer:
Verified
Q16: Give the formal charge on the phosphorus
Q17: Of the bonds marked, which bond is
Q18: Given that the acetate anion has the
Q19: Add valence electrons to the structures so
Q20: What is the geometry of the borohydride
Q21: Complete the electron configuration diagram below for
Q22: Assume that the structure of BF3 (boron
Q23: Consider this structure:
Q24: Consider this structure: Q26: Consider this structure:
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents