Urea is a water-soluble product of nitrogen metabolism. How many hydrogen bonds can one urea molecule donate to surrounding water molecules? 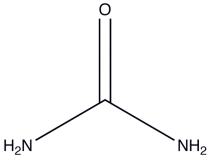
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer:
Verified
Q11: Due to the formation of hydrogen bonds,
Q12: Which of the following explains the interactions
Q13: The polarity of the O-H bond is
Q14: Which of the following is a physical
Q15: Which of the following functional groups has
Q17: In an aqueous solution, if the [OH-]
Q18: What is the [H+] of an aqueous
Q19: What would be the resulting pH if
Q20: What would be the resulting pH if
Q21: Which of the following would be the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents