The figure here shows the distribution of molecular speeds of a gas for three temperatures T1, T2 and T3. The ranking of the temperatures is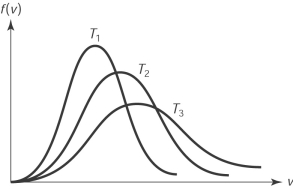
A) T1 < T2 < T3.
B) T1 > T2 > T3.
C) T2 < T1< T3.
D) T2 > T1> T3.
E) T1= T2 = T3.
Correct Answer:
Verified
Q33: The temperature (in kelvins) of a
Q34: An ideal gas is slowly expanding
Q35: If the rms speed in a particular
Q36: The equivalent of the root-mean-square speed for
Q37: There are 5.00
Q39: The figure here shows the distribution
Q40: The molecules of a five-particle gas have
Q41: The molecules of a five-particle gas have
Q42: The molecules of a five-particle gas have
Q43: The mass of a deuterium (diatomic) molecule
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents