Hydrogen sulfide ion, HS-, can react differently depending on the acidity of the solution in which it is present.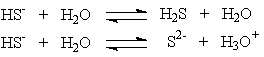 These two solutions show that the
These two solutions show that the
A) hydrogen sulfide ion is an acid.
B) hydrogen sulfide ion is a base.
C) hydrogen sulfide ion is amphoteric.
D) hydrogen sulfide ion can only react with water.
Correct Answer:
Verified
Q4: A conjugate acid-base pair
A) is related by
Q5: Which of the following is the conjugate
Q6: In the reaction: Q7: In the reaction: Q8: Which of the following is the conjugate Q10: Which of the following is the conjugate Q11: Which of the following is the conjugate Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()

