Which of the following is the correct statement concerning the equation below? 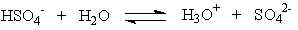
A) HSO4- is the conjugate base of SO4-
B) HSO4- is the conjugate base of H3O+
C) H2O is the conjugate base of HSO4-
D) SO42- is the conjugate base of HSO4-
Correct Answer:
Verified
Q8: Which of the following is the conjugate
Q9: Hydrogen sulfide ion, HS-, can react differently
Q10: Which of the following is the conjugate
Q11: Which of the following is the conjugate
Q12: In the equation: Q14: Whenever an equilibrium constant, Keq, has a Q15: When a reaction is at equilibrium, Q16: Choose the equilibrium constant that indicates the Q17: The equation: Q18: The equilibrium constant, Keq, for![]()
A) it![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents