The pH of blood is maintained at 7.35-7.45 by the following buffer system: The H2CO3 is produced by the reaction of CO2 with water according to the equation:
The H2CO3 is produced by the reaction of CO2 with water according to the equation: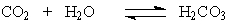 When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
A) The pH will increase.
B) The pH will decrease.
C) The pH will remain the same.
D) It is impossible to predict the effect.
Correct Answer:
Verified
Q49: The pH of blood is held reasonably
Q50: Excessive use of antacids can lead to
A)
Q51: The ethylammonium ion, CH3CH2NH3+ has a pKa
Q52: The carbonic acid/bicarbonate buffer is important in
Q53: The Ka for the reaction of acetic
Q55: Antacids may contain which ion to reduce
Q56: Acids and bases can react with and
Q57: HNO3 can act as acid and
Q58: The higher the numerical value of an
Q59: The greater the hydronium ion concentration, the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents