The Ka for the reaction of acetic acid and water shown below is 1.8 x 10-5.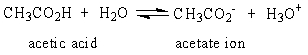 Which of the following statements is true at pH 7?
Which of the following statements is true at pH 7?
A) there is much more acetic acid than acetate ion
B) there is more acetate ion than acetic acid
C) the concentration of acetate ion is equal to that of acetic acid
D) the pH is lower than pKa of acetic acid
Correct Answer:
Verified
Q48: What volume of 0.200 M HCl is
Q49: The pH of blood is held reasonably
Q50: Excessive use of antacids can lead to
A)
Q51: The ethylammonium ion, CH3CH2NH3+ has a pKa
Q52: The carbonic acid/bicarbonate buffer is important in
Q54: The pH of blood is maintained at
Q55: Antacids may contain which ion to reduce
Q56: Acids and bases can react with and
Q57: HNO3 can act as acid and
Q58: The higher the numerical value of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents