Consider these metal ion/metal standard reduction potentials 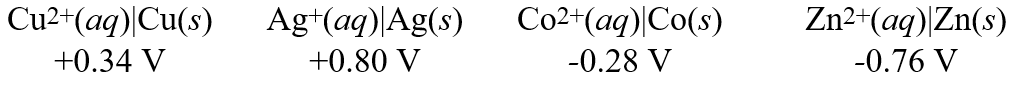 Based on the data above, which one of the species below is the best reducing agent?
Based on the data above, which one of the species below is the best reducing agent?
A) Co(s)
B) Zn(s)
C) Cu2+(aq)
D) Cu(s)
E) Ag(s)
Correct Answer:
Verified
Q18: Using the standard reduction potentials::
Q19: For the reaction, 2 Cr2+(aq)+ Cl2(g)
Q20: The cell described by the net reaction:2U(s)+
Q21: Consider this electrochemical cell:Pt | Pu3+(aq),
Q22: The cell described by the reaction,2 Co3+(aq)+
Q24: Using the standard reduction potentials
Q25: Using the standard reduction potentials
Q26: Consider these metal ion/metal standard reduction potentials
Q27: Consider these metal ion/metal standard reduction potentials
Q28: Consider these metal ion/metal standard reduction potentials
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents