Using the standard reduction potentials 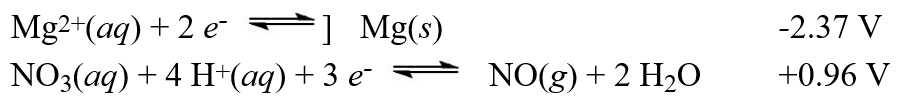 Calculate the value of E°cell for the cell with the reaction:
Calculate the value of E°cell for the cell with the reaction:
3 Mg(s) + 2 NO3-(aq) + 8 H+(aq) 3 Mg2+(aq) + 2 NO(g) + 4 H2O
A) +1.41 V
B) -1.41 V
C) +3.33 V
D) +8.46 V
E) -8.46 V
Correct Answer:
Verified
Q19: For the reaction, 2 Cr2+(aq)+ Cl2(g)
Q20: The cell described by the net reaction:2U(s)+
Q21: Consider this electrochemical cell:Pt | Pu3+(aq),
Q22: The cell described by the reaction,2 Co3+(aq)+
Q23: Consider these metal ion/metal standard reduction potentials
Q25: Using the standard reduction potentials
Q26: Consider these metal ion/metal standard reduction potentials
Q27: Consider these metal ion/metal standard reduction potentials
Q28: Consider these metal ion/metal standard reduction potentials
Q29: Consider these metal ion/metal standard reduction potentials
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents