A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 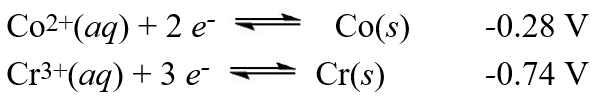 What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
A) -88.8 kJ
B) -178 kJ
C) -266 kJ
D) -295 kJ
E) -590 kJ
Correct Answer:
Verified
Q36: The Faraday constant is equal to the
Q37: One mole of electrical charge contains
A)4.184 joules.
B)3,600
Q38: A galvanic cell is composed of these
Q39: A galvanic cell is composed of
Q40: A galvanic cell is composed of
Q42: A galvanic cell is composed of
Q43: A galvanic cell is composed of
Q44: A galvanic cell is composed of
Q45: Using the standard reduction potentials
Q46: Using these metal ion/metal standard reduction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents