A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 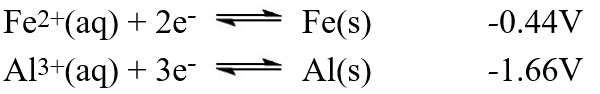 What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
A) -806 kJ
B) -1.22 × 103 kJ
C) -706 kJ
D) -540 kJ
E) -600 kJ
Correct Answer:
Verified
Q37: One mole of electrical charge contains
A)4.184 joules.
B)3,600
Q38: A galvanic cell is composed of these
Q39: A galvanic cell is composed of
Q40: A galvanic cell is composed of
Q41: A galvanic cell is composed of
Q43: A galvanic cell is composed of
Q44: A galvanic cell is composed of
Q45: Using the standard reduction potentials
Q46: Using these metal ion/metal standard reduction
Q47: Using the reduction potentials given, calculate the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents