Table of standard electrode potentials 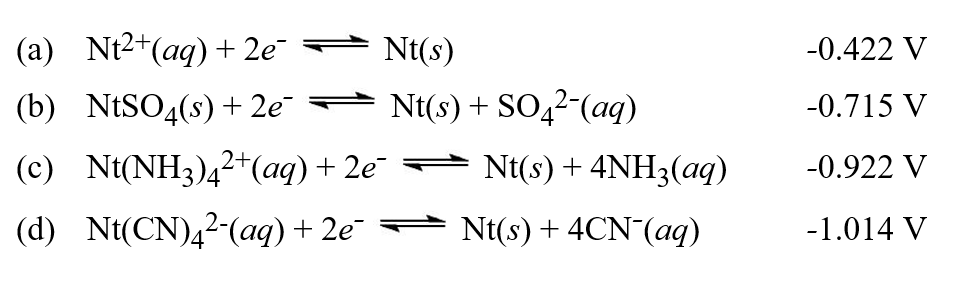
-Using a table of standard electrode potentials decide which of the following statements is completely true.(Note that some imaginary elements are being used in these questions) .
A) Nt2+ can oxidize Cu2+, and Al3+ can reduce H+.
B) Br2 can oxidize Ni, and H2 can reduce Cl-.
C) Cu2+ can oxidize H2, and Nt can reduce Cl2.
Correct Answer:
Verified
Q125: When adding half reactions and canceling out
Q126: Lithium metal can be produced readily by
Q127: During the electrolysis of water, oxygen gas
Q128: When an electrical current is passed through
Q129: If a brine bath is stirred during
Q131: Table of standard electrode potentials 
Q132: Which one of the following, when added
Q133: Use the standard reduction potentials below to
Q134: The equation for the chemical reaction from
Q135: Calculate the value of the equilibrium constant,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents