Calculate the value of the equilibrium constant, Kc, for the reactionNt(CN)42-(aq)+ SO42-(aq)  NtSO4(s)+ 4CN-(aq)Hint: Use these standard potentials in answering this question. (Note that the imaginary element Nt is being used).
NtSO4(s)+ 4CN-(aq)Hint: Use these standard potentials in answering this question. (Note that the imaginary element Nt is being used). 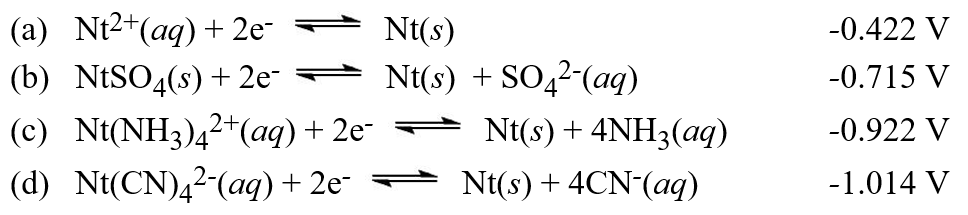
Correct Answer:
Verified
Q130: Table of standard electrode potentials 
Q131: Table of standard electrode potentials 
Q132: Which one of the following, when added
Q133: Use the standard reduction potentials below to
Q134: The equation for the chemical reaction from
Q136: The solubility product constant for NtCrO4 is
Q137: What is value of the formation constant
Q138: Given the following standard reduction potentials,
Q139: Calculate the cell emf for the following
Q140: The measured voltage of the cell Pt(s)|
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents