Given the following standard reduction potentials, 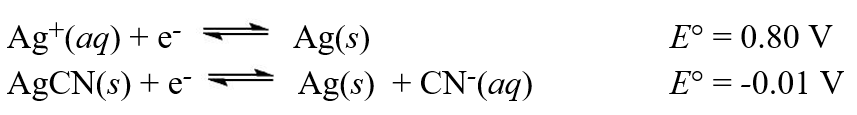 calculate the solubility product (Ksp)of AgCN at 25°C.
calculate the solubility product (Ksp)of AgCN at 25°C.
Correct Answer:
Verified
The first step in solving this ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q133: Use the standard reduction potentials below to
Q134: The equation for the chemical reaction from
Q135: Calculate the value of the equilibrium constant,
Q136: The solubility product constant for NtCrO4 is
Q137: What is value of the formation constant
Q139: Calculate the cell emf for the following
Q140: The measured voltage of the cell Pt(s)|
Q141: An old classmate who works for Eagle
Q142: Note: Imaginary elements are used in the
Q143: Note: Imaginary elements are used in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents