Using these bond energies, ?H°: 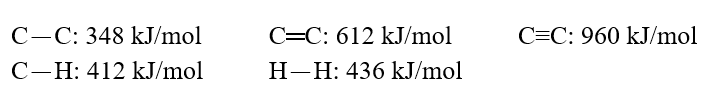 Calculate the value of H° of reaction for H2C.H2(g) + H2(g) CH3-CH3(g)
Calculate the value of H° of reaction for H2C.H2(g) + H2(g) CH3-CH3(g)
A) -560 kJ/mol
B) -124 kJ/mol
C) -388 kJ/mol
D) +224 kJ/mol
E) -212 kJ/mol
Correct Answer:
Verified
Q57: The thermochemical equation representing one process
Q58: Determine the equilibrium constant Kp at
Q59: Determine the equilibrium constant Kp at
Q60: The equilibrium constant at 427°C for
Q61: The equilibrium constant at 25°C for
Q63: Using these bond energies, ?H°:
Q64: Using these bond energies,
Q65: According to the first law of thermodynamics
Q66: According to the first law of thermodynamics
Q67: The _ phase of a substance will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents