Using these bond energies, ?H°: 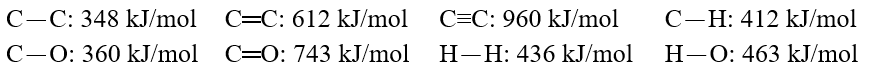 Calculate the value of H° of reaction for O.C.O(g) + 3 H2(g) . CH3-O-H(g) + H-O-H(g)
Calculate the value of H° of reaction for O.C.O(g) + 3 H2(g) . CH3-O-H(g) + H-O-H(g)
A) -191 kJ/mol
B) +272 kJ/mol
C) -272 kJ/mol
D) -5779 kJ/mol
E) +5779 kJ/mol
Correct Answer:
Verified
Q58: Determine the equilibrium constant Kp at
Q59: Determine the equilibrium constant Kp at
Q60: The equilibrium constant at 427°C for
Q61: The equilibrium constant at 25°C for
Q62: Using these bond energies, ?H°:
Q64: Using these bond energies,
Q65: According to the first law of thermodynamics
Q66: According to the first law of thermodynamics
Q67: The _ phase of a substance will
Q68: The reaction N2(g)+ 3H2(g)→ 2NH3(g)would result in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents