A 10-mm cube of copper metal is placed in 250 mL of 12 M nitric acid at 25°C and the reaction below occurs: Cu(s) + 4H+(aq) + 2NO3-(aq) Cu2+(aq) + 2NO2(g) + 2H2O(l) At a particular instant in time, nitrogen dioxide is being produced at the rate of 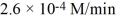 . At this same instant, what is the rate at which hydrogen ions are being consumed?
. At this same instant, what is the rate at which hydrogen ions are being consumed?
A) 1.3 × 10-4 M/min
B) 5.2 × 10-4 M/min
C) 2.6 × 10-4 M/min
D) 1.0 × 10-3 M/min
E) 6.5 × 10-5 M/min
Correct Answer:
Verified
Q4: Nitrogen monoxide reacts with bromine at
Q5: Nitrogen monoxide reacts with chlorine at
Q6: In a reaction described by the
Q7: In a reaction described by the
Q8: Cyclobutane, C4H8, decomposes as shown: C4H8(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents