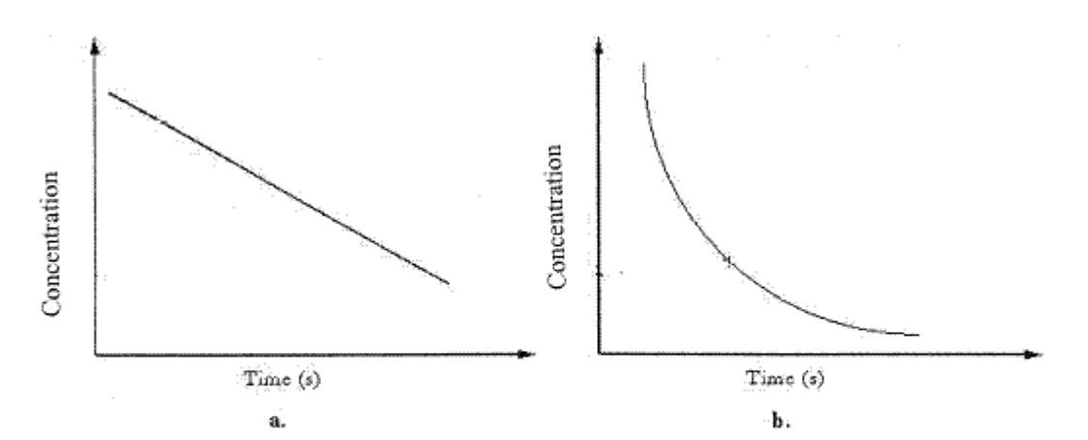
 The average reaction rate over a time period in graph b can be found by
The average reaction rate over a time period in graph b can be found by
A) taking the average of the rate every 100 s.
B) drawing a line tangent to the curve at the initial and final times, and then averaging.
C) drawing a line tangent to the curve at that point.
D) subtracting the final concentration from the initial concentration and dividing by the time interval.
E) subtracting the final time from the initial time and dividing by the concentration interval.
Correct Answer:
Verified
Q5: Nitrogen monoxide reacts with chlorine at
Q6: In a reaction described by the
Q7: In a reaction described by the
Q8: Cyclobutane, C4H8, decomposes as shown: C4H8(g)
Q9: A 10-mm cube of copper metal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents