The reaction, A + 2B products, was studied. A timer was started when reagents A and B were mixed, and stopped when a specific quantity of product C accumulated. The data contains initial amounts of reactants used and the time needed to reach the specific quantity of C. Based on the data below, we can conclude that 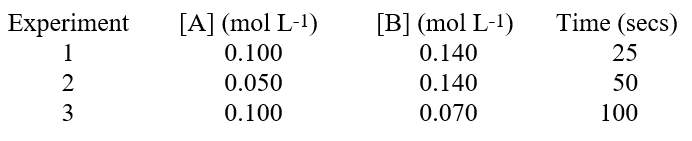 Hint: Remember reaction rate is proportional to 1/time.
Hint: Remember reaction rate is proportional to 1/time.
A) the reaction is first order with respect to substance A.
B) the reaction is zero order with respect to substance A.
C) the reaction is one-half order with respect to substance A.
D) the reaction is second order with respect to substance A.
E) the reaction is third order with respect to substance B
Correct Answer:
Verified
Q23: The units of the rate constant for
Q24: The units of the rate constant for
Q25: Nitric oxide reacts with bromine at
Q26: For the reaction, 3B + C
Q27: Given the reaction, aA + bB
Q29: For the reaction, 2M + 2N
Q30: For the reaction, A + 2B
Q31: For the reaction, A + 2B
Q32: The reaction, 2A2X4(g)
Q33: The data below were obtained in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents